日本電子テクノサービス株式会社のスタートとなった翻訳サービスを原点として、現在は主にグループ会社である日本電子株式会社の製品マニュアルの制作から翻訳までのドキュメント制作サービスを行っております。
製品の一部として世界中のお客様にマニュアルが届き、科学の進歩と社会の発展に貢献できることに大きな喜びを感じております。
製品マニュアル以外に営業仕様書、社内技術情報、サービス員向け作業要領書などの制作を手がけております。
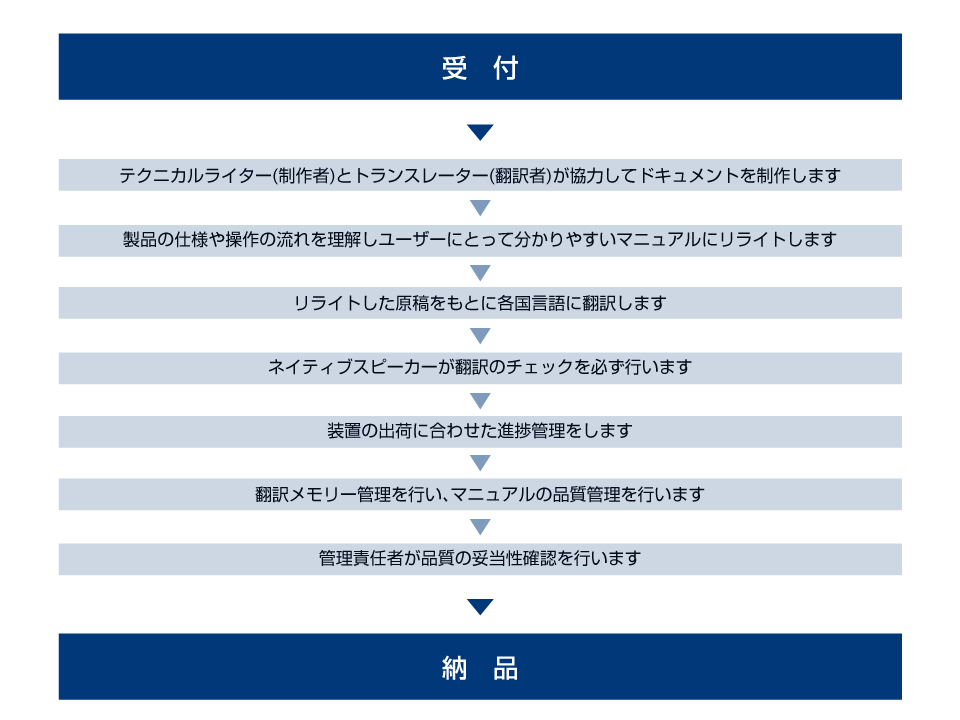
サービスの流れ
使用しているツール
Microsoft Office、Adobe Creative Cloud、Trados Studioなどお仕事紹介

テクニカルライター(制作者)
テクニカルライターは、技術部門から提供された取扱説明書の素案を、実際に製品ユーザーにお渡しできる形に仕上げる作業を行っています。製品の操作やしくみに関して、主なユーザーである研究者、専門家の皆様にとって必要かつ十分な内容となるよう心掛けて文章の編集を行っています。

トランスレーター(翻訳者)
日本電子テクノサービスの翻訳者は、製品に関する情報を確かな翻訳で伝えることを目指しています。世界中にいる製品のユーザーや日本電子グループのサービス員・営業員など私たちの翻訳の読み手のことを第一に考えながら、日々翻訳品質の向上に取り組んでいます。
翻訳サービスについて
主な翻訳分野科学・物理・化学・生物学・技術・工学・工業・産業・法律・安全規格、品質規格・理化学工業・産業プラント・環境プラント・ものづくり技術などの翻訳分野で、 正確且つ自然な和英中語の翻訳を、リーズナブルな価格でお届けいたします。
料金についてはお問い合わせください。
弊社が翻訳のお手伝いをしているWEBサイトの例


TEL. 042-542-2127
日本電子テクノサービス ドキュメント制作部
スタッフインタビュー

2019年11月入社 T.I
テクニカルライターとしてパート入社。グループ内製品のマニュアル制作に携わっている。
時短勤務を経て、フルタイムへ移行。2023年4月より社員に登用。

